On October 7, National Health Commission of the People’s Republic of China (NHC) issued an announcement (No. 8 of 2023) on the approval of 15 “Three New Foods”, including 4 new food raw materials, 6 new food additives, and 5 food-related products. Details are as follows:
New food raw materials (4 types)
1. Peach gum
| Name | Peach gum | |
| Basic information | Source: Prunus persica (L.) Batsch | |
| Brief introduction of the production process | Prepared through picking, sorting, airing, cleaning, drying, and other processes by using the gum secreted from Prunus persica (L.) Batsch. | |
| Recommended intake | ≤30 g/day | |
| Quality requirements | Crude polysaccharide, g/100 g | ≥ 60 |
| Moisture, g/100 g | ≤ 15 | |
| Ash, g/100 g | ≤ 2 | |
| Other information | The labels and instructions shall bear a statement indicating that they should not be consumed by infants, pregnant women and lactating women, along with the recommended intake.Food safety indicators must meet the following requirements: | |
| Pb, mg/kg | ≤ 0.5 | |
| As, mg/kg | ≤ 0.5 | |
| Ochratoxin A, μg/kg | ≤ 5.0 | |
| Coliforms, CFU/g | ≤ 100 | |
| Mould and Yeast, CFU/g | ≤ 150 | |
| Staphylococcus aureus, /25g | 0 | |
| Salmonella, /25g | 0 |
2. Tiger nut
| Name | Tiger nut |
| Basic information | Source: Cyperus esculentus L. var. sativus Boeck.Edible part: stem tubers |
| Other information | Food safety indicators shall comply with the current national food safety standard for nuts and seeds. |
3. Leuconostoc mesenteroides subsp. cremoris
| Name | Leuconostoc mesenteroides subsp. cremoris |
| Other information | Being included in the List of Strains that Can be Used in Food, and approved for the use in fermentation processing of milk and dairy products, fruit and vegetable products, and grain products (excluding infants and young children food).Food safety indicators must meet the following requirements: |
| Pb, (on dry basis), mg/kg | ≤ 1.0 |
| As, (on dry basis), mg/kg | ≤ 1.5 |
| Salmonella, /25g (mL) | 0 |
| Staphylococcus aureus, /25g (mL) | 0 |
| Listeria monocytogenes, /25g (mL) | 0 |
4. Pyrroloquinoline quinone disodium (PQQ) salt
| Name | Pyrroloquinoline quinone disodium (PQQ) salt | |
| Basic information | CAS: 122628-50-6Molecular formula: C14H4N2Na2O8Structure: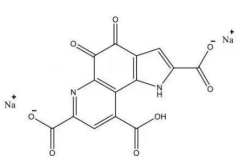 Molecular weight: 374.17 Molecular weight: 374.17 | |
| Brief introduction of the production process | Prepared through fermentation, extraction, purification, crystallization, drying, and other processes by using Methylovorus glucosotrophus as the production strain. | |
| Recommended intake | ≤20 mg/day | |
| Quality requirements | Property: | Reddish brown powder |
| Pyrroloquinoline quinone disodium (PQQ) salt content (on dry basis), g/100 g | ≥ 98.0 | |
| Moisture, g/100 g | ≤ 12.0 | |
| Other information | Application scope and maximum usage level: beverages (40 mg/kg, solid beverages are converted according to the mass of liquid after preparation).The labels and instructions shall bear a statement indicating that they should not be consumed by infants, pregnant women and lactating women, along with the recommended intake.Test methods for Pyrroloquinoline quinone disodium (PQQ) salt are given in Appendix.Food safety indicators must meet the following requirements: | |
| Pb, mg/kg | ≤ 0.5 | |
| As, mg/kg | ≤ 1.0 | |
| Cd, mg/kg | ≤ 0.1 | |
| Hg, mg/kg | ≤ 0.1 | |
| Total plate count, CFU/g | ≤ 1000 | |
| Coliforms, MPN/g | ≤ 3.0 | |
| Mould and Yeast, CFU/g | ≤ 100 | |
| Staphylococcus aureus, /25g | 0 | |
| Salmonella, /25g | 0 |
New food additives (6 types)
1. New food enzyme (1 type)
| No. | Enzyme | Source | Donor |
| 1 | Serine protease | Bacillus licheniformis | Nocardiopsis prasina |
* The quality specifications of the enzyme shall meet the requirements in the National Food Safety Standard – Food Additives, Enzymes (GB 1886.174).
2. New food nutrition enhancers (3 types)
(1) Name: Magnesium lactate
Application scope and maximum usage level: It shall be consistent with the requirements of magnesium in the National Food Safety Standard – Standard for the Use of Food Nutrition Enhancer (GB 14880).
Quality specifications: Applicable to the new food nutrition enhancer magnesium lactate, which is prepared through the reaction of lactic acid and magnesium oxide (or magnesium carbonate).
(2) Name: 2’-fucosyllactose, 2’-FL
Application scope and maximum usage level:
| Category number | Food name/category | Usage level | Note |
| 01.03.02 | Modified milk powder (limited to milk powder for children) | 0.7-2.4 g/L (Count as the state of ready to eat, for powdery products, the level of use should be increased by times of brewing) | When mixed with Lacto-N-neotetraose (LNnT), oligo-galactose, oligofructose, polyfructose and raffinose, the total amount of 2′-fucosyllactose must not exceed 64.5 (g/kg). |
| 13.01.01 | Infant formula foods | ||
| 13.01.02 | Older infants and young children formula foods | ||
| 13.01.03 | Infant formula foods for special medical purpose |
Quality specifications: Applicable to the new food nutrition enhancer 2’-fucosyllactose (2’-FL), which is prepared through fermentation, purification, drying, and other processes using lactose as a raw material. The production strain of 2’-FL should undergo safety assessment and comply with the requirements outlined in Appendix C.
Appendix C Information on the production strain of 2’-fucosyllactose
| Nutrition enhancer | Source | Donor |
| 2’-fucosyllactose | Coli K12 DH1 MDO | (Helicobacter spp.)a |
| E. coli K-12 MG1655 | (Helicobacter spp.)a | |
| E. coli BL21 (DE3) | (Neisseria spp.)a |
a is the donor of α-1, 2-fucosyltransferase
(3) Name: Lacto-N-neotetraose, LNnT
Application scope and maximum usage level:
| Category number | Food name/category | Usage level | Note |
| 01.03.02 | Modified milk powder (limited to milk powder for children) | 0.2-0.6 g/L (Count as the state of ready to eat, for powdery products, the level of use should be increased by times of brewing) | When mixed with 2’-fucosyllactose, oligo-galactose, oligofructose, polyfructose and raffinose, the total amount must not exceed 64.5 (g/kg). |
| 13.01.01 | Infant formula foods | ||
| 13.01.02 | Older infants and young children formula foods | ||
| 13.01.03 | Infant formula foods for special medical purpose |
Quality specifications: Applicable to the new nutrition enhancer Lacto-N-neotetraose (LNnT), which is prepared through fermentation, purification, drying, and other processes using lactose as a raw material. The production strain of LNnT should undergo safety assessment and comply with the requirements outlined in Appendix D.
Appendix D Information on the production strain of Lacto-N-neotetraose
| Nutrition enhancer | Source | Donor |
| Lacto-N-neotetraose | E. coli K-12 DH1 MDO | (Neisseria spp.)a and (Helicobacter spp.)b |
a is the donor of β-1,3-N-acetylglucosamine transferase
b is the donor of β-1,4-galactosyltransferase
3. New food additives with expanded scope (2 types)
| No. | Name | Function | Category number | Food name/category | Maximum level (g/kg) | Note |
| 1 | Calcium lactate | Stabilizer and coagulant, acidity regulator | 04.02.02.03 | Pickled vegetables | 10.0 | – |
| 04.02.02.04 | Canned vegetables | 3.0 | ||||
| 2 | Sanzan gum | Thickener, stabilizer and coagulant | 01.01.03 | Modified milk | 0.5 | – |
| 14.03.03 | Mixed protein drinks | 0.75 | Count as the state of ready to drink, increase the usage of solid drinks according to the dilution ratio. | |||
| 14.08 | Flavored drinks | 0.5 |
New food-related products (5 types)
1. Food contact additives with expanded scope (3 types)
| No. | Name | CAS number | Application scope | Maximum level (%) |
| 1 | C.I. pigment black 7; Carbon black | 1333-86-4 | Plastic: PEEK | 0.5 |
| 2 | Copolymer of acrylamide with methacryloyloxyethyltrimethylammonium chloride, itaconic acid, and N, N ‘- methylene bisacrylamide | 214495-32-6 | Paper and paperboard | 1.5(Count as the dry weight) |
| 3 | 2- (Vinyloxy) -1,2,3-propanecarboxylic acid tributylester | 77-90-7 | Ink with indirect contact with food | 10 |
2. New food contact resin (2 types)
| No. | Name | CAS number | Range of application | Maximum level (%) |
| 1 | 1,4-Benzenedicarboxylic acid, polymer with sebacic acid and 1,2-ethanediol | 25067-21-4 | Coating and coating film | Appropriate level |
| 2 | Polymers of methacrylic acid with butyl methacrylate, ethyl acrylate and methyl methacrylate, and polymers of hydroquinone with 4,4-methylenebis (2,6-dimethylphenol) and chloromethylepoxyethane with N, N-dimethylethanolamine | – | Coating and coating film | 20(Count as the coating formula) |
(Source: CIRS Group)
Sign Up to Receive China Updates Weekly Newsletter for FREE, HERE
Visit HPA-China’s Information Hub, HERE
