Recent reports indicate that multiple Chinese e-commerce platforms have released notices stating that NMN (Nicotinamide Mononucleotide) food/supplements produced in certain countries and regions will be prohibited from being sold on cross-border e-commerce(CBEC) platforms in China.
Banned Sales Regions:
food products containing NMN ingredients produced in the following countries and regions: Taiwan, China; Macau, China; United States; Australia; Germany; Switzerland; United Kingdom; Singapore; Spain; Netherlands; Hungary; Denmark; Sweden; Czech Republic; Norway; Poland; Austria; France.
Permitted Sales Regions:
NMN products that have obtained food sales permits in the following regions can still be sold under specific conditions: Canada, Hong Kong, China, and Japan.
Customs Measures and Risk Warnings for Food Products Containing NMN Ingredients via CBEC:
1. Strict Inspection: Customs will focus on verifying the behavior of smuggling NMN-containing food products through other countries or regions.
2. Price Verification and Tax Costs: NMN products imported through direct mail channels will face stricter price verification and higher tax rates.
3. Product Standards and Quality Safety: Regulatory authorities are particularly focused on the product standards and quality safety of NMN supplements, and non-compliant products will be strictly investigated and disposed of.
Regulatory Background
In January 2021, while investigating a batch of illegal NMN products, China’s State Administration for Market Regulation issued a letter on “Investigating Illegal Operations of ‘Anti-aging Drugs’,” which clearly stated that NMN has not yet obtained permits for drugs, health foods, food additives, or new food ingredients in China, and cannot be used in food production and operations.
In January 2023, the National Health Commission officially accepted the application for NMN as a new food additive. However, on May 9, 2023, the National Health Commission issued a notice rejecting the administrative licensing of 21 newly reported food additives, including NMN. This means that at least for now, NMN does not have legal support for application in the domestic food or drug fields. Adding NMN to products would be considered illegal addition.
On April 17, 2024, China’s General Administration of Customs further issued a risk alert, reminding consumers to be cautious when purchasing products containing NMN ingredients.

Currently, on e-commerce platforms such as Taobao and JD.com, there are still a large number of NMN products being sold, with origins including Japan, the United States, and Hong Kong, China. However, some bonded warehouses such as the Ningbo Beilun Customs Clearance Warehouse have stated that they can no longer ship these products.
Some products with “NMN” on their packaging have been blurred out on the Tmall International flagship store of Kingdomway (one of the key players), and the bottom of the product detail page indicates “This product is not supported for sale in the current region.” Inquiries with customer service revealed that the “NAD+” products are currently out of stock and the brand is working on replenishment, and the representative stated that the products are currently banned from sale.
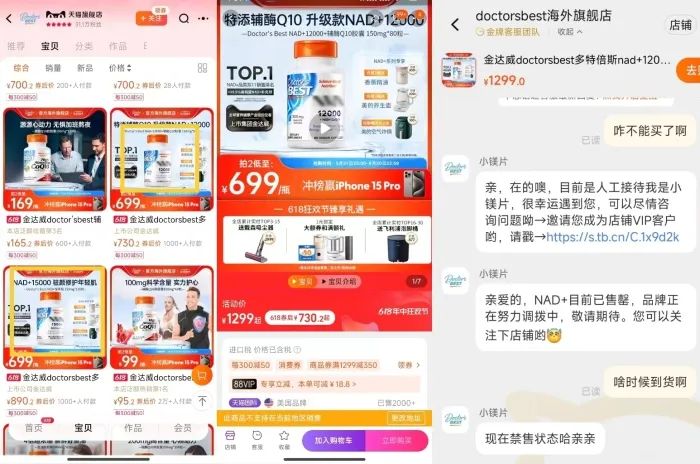
*Images are from Tmall International
Sign Up to Receive China Updates Weekly Newsletter for FREE, HERE
Visit HPA-China’s Information Hub, HERE
