Jul 05, 2024 from CIRS by Jiaxin Liu
ChinaHealth FoodFood and Food Related Products
According to information released by the Center for Food Evaluation (CFE) and the Special Food Information Query Platform, a total of 720 health food (dietary supplement) registration approvals were issued in the first half of 2024, 253 of which are new health food products.
CIRS Group has conducted a detailed summary of these 253 new products and analyzed them from multiple perspectives.
Overview of health food registration approvals issued in the first half of 2024
Among the 720 registration approvals, 253 were new products, accounting for 35.14% of the total. Additionally, there were 467 other approvals (renewal, change of registration, etc.).

The number of health food registrations (by approval date)
As shown in Figure 2, in January, February, April, and May, there are 89, 68, 49, and 46 new products registration approvals issued, respectively. No new product approvals were released in the other months.
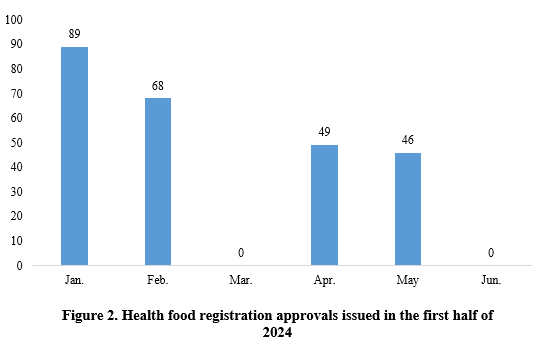
Note 1: One new product did not have precise approval date matched and therefore is not included in the statistics.
The number of approved products in different regions
The 253 approved new products were from 29 provinces (municipalities and/or autonomous regions). Beijing, Guangdong and Shandong ranked top three with the number of 56, 29 and 21 respectively, accounting for 22.13%, 11.46% and 8.30% of the total.
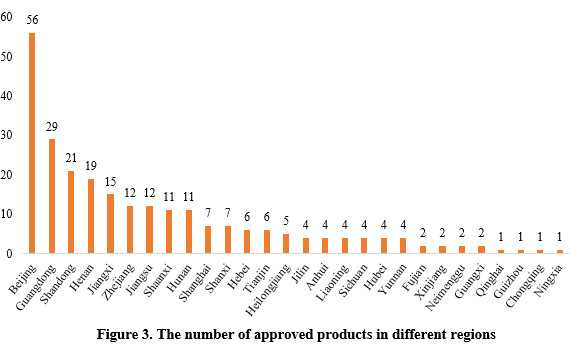
Note 2: If the applicant includes more than one enterprises and comes from different provinces, each province is included in the statistics.
Enterprises obtaining health food registration certificates
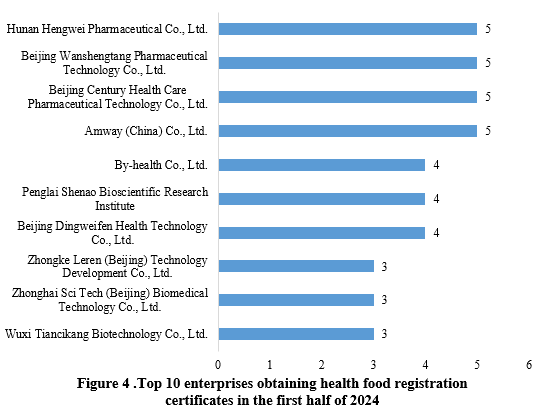
Hunan Hengwei Pharmaceutical Co., Ltd., Beijing Wanshengtang Pharmaceutical Technology Co., Ltd., Beijing Century Health Care Pharmaceutical Technology Co., Ltd. and Amway (China) Co., Ltd., each obtained 5 health food registration approvals in the first half of 2024, sharing the top position. The top ten enterprises obtaining new registration certificates in the first half of 2024 are shown in Figure 4.
The number of approved products with different dosage forms
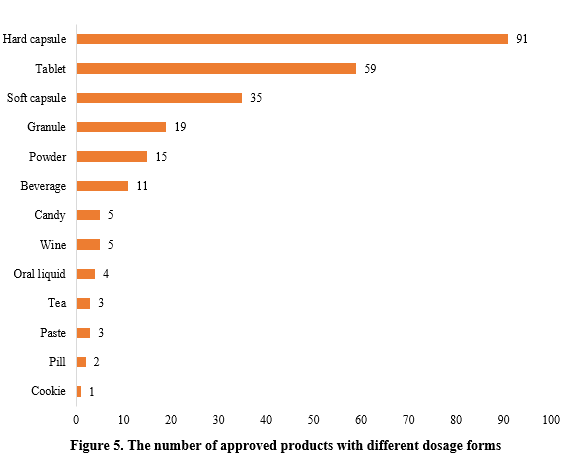
The dosage forms of approved new products include capsules, tablets, powder, oral liquid, etc. Among them, hard capsules topped the list with 91 products, accounting for 35.97% of the total, followed by tablets with 59 products, accounting for 23.32%. In addition to the common health food dosage forms, some food forms such as biscuits and candies also received registration approvals.
The number of approved products with different health functions
Single health function
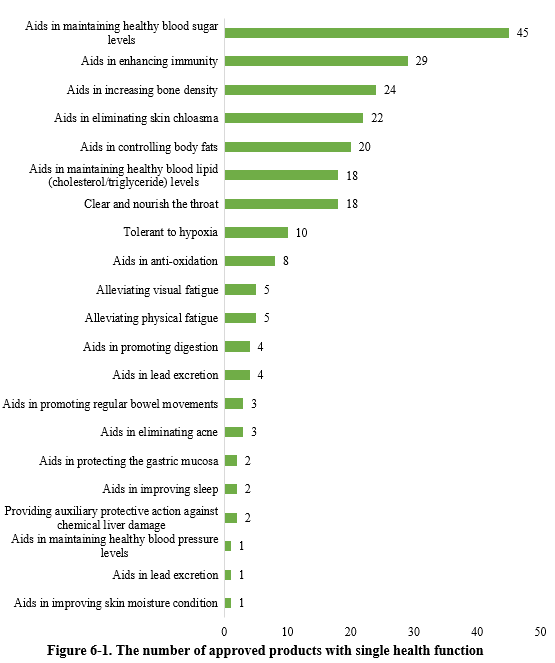
As shown in Figure 6-1, there were 227 new products approved with single health function in the first half of 2024. Among these, products with the health function of “aids in maintaining healthy blood sugar levels” received the largest number of approvals, with 45 products.
Two health functions
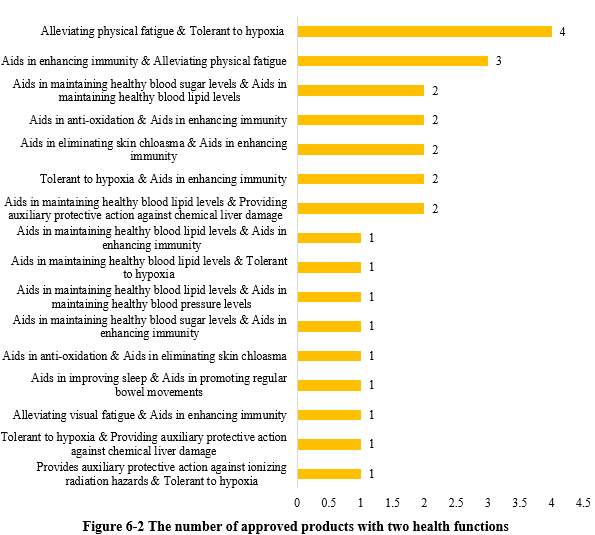
Except for those new products with single health function, there were 26 products approved with two health functions (shown in Figure 6-2). Among these, the most common claim was “relieving physical fatigue and improving tolerance to hypoxia”, with 4 products.
CIRS Comments
New health food products approvals only accounted for 35.14% of the total registration approvals issued in the first half of 2024, indicating that the review process of new products is still in a recovery phase. Moreover, companies are increasingly focusing on the health needs of consumers rather than opting solely for traditional health functions.
In 2023, the issuance of Directory of Health Functions Available to be Claimed by Health Food – Non-nutrition Supplements (2023 Version) officially began the changing of registration certificates for health foods approved by former Ministry of Health (MOH). CIRS Group has received many inquiries from enterprises about this process, and we suggest that those in need prepare the materials early according to the Key Points for the Review of the Change of Registration Certificate for Health Foods with No Validity Period and No Technical Requirements in Production and Sale (Draft), so that once the official documents are released, they can swiftly proceed with necessary procedures. (Source: CIRS Group)
Sign Up to Receive China Updates Weekly Newsletter for FREE, HERE
Visit HPA-China’s Information Hub, HERE
