Recently, the newly revised Food Label Supervision and Administration Measures (hereinafter referred to as the “Measures”) and the National Food Safety Standard General Rules for Prepackaged Food Labels (GB 7718-2025) were officially released and will take effect simultaneously on March 16, 2027. These new regulations standardize various labeling requirements for food products and further clarify the requirements for special food labels, including health food.
The “Measures” states that “If a trademark name other than the one appearing in the product name is used on the label, its area must not exceed one-fourth of the product name area. This additional trademark must be smaller than the trademark in the product name. It must not be used together with the product name in a way that may mislead consumers into believing it is part of the product name.” This clause aims to regulate OEM trademark in health food labeling.
For health foods with multiple independent packages or multi-layer packaging, the “Measures” provide specific labeling requirements for the outer packaging (minimum sales unit) and the inner packaging.
Outer Packaging (minimum sales unit)
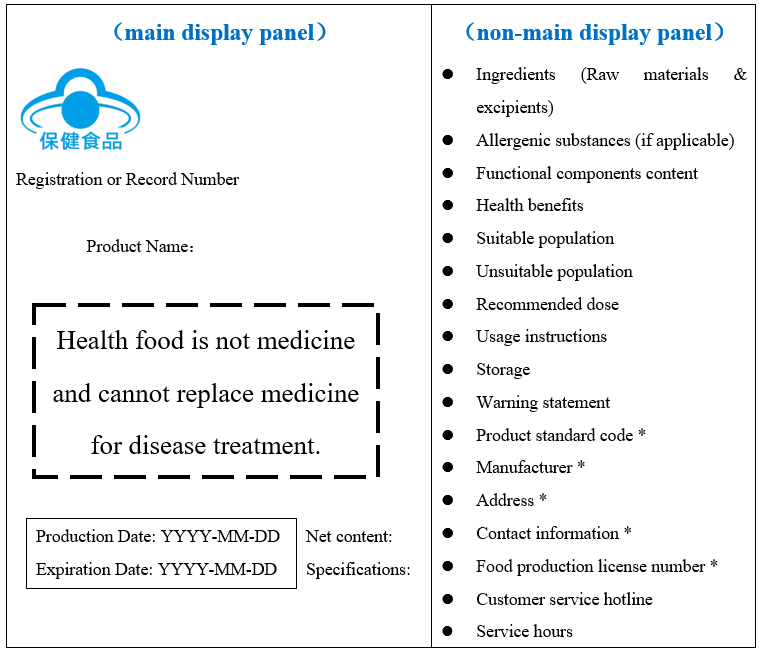
Note:
- Items marked with* are required for domestic products. For imported products, labels must include: Country of origin, importer/agent name, address, contact information, and foreign manufacturer’s China registration number.
- Nutritional supplements must explicitly state “Nutritional Supplement” on the label.
- For small packages (<35cm²), the label must at least include: Registration/record number, health food logo, manufacturer name, food production license number, production & expiration dates, and the warning statement.
- Product names must not be split (e.g., brand name, generic name, and attribute name must be displayed in the same font, color, and size).
- Production & expiration dates must be in a dedicated area and must not be easily removed. If not on the main display panel, the location should be clearly indicated.
Inner Packaging (non-independent sales unit)

CIRS Opinion
China classifies health food as special food subject to strict supervision. Compliance regulations are stringent, and product labels, being a key part of marketing, are closely monitored by government agencies. In recent years, China has introduced multiple health food labeling regulations, including: Guidelines for Naming Health Food, Guidelines for Health Food Warning Statements, and Guidelines for Standardizing Health Food Logos. The newly issued labeling regulations (GB 7718-2025 and the “Measures”) provide a 2-year transition period before full enforcement on March 16, 2027. Businesses should familiarize themselves with the new labeling rules early, adjust product labels in advance, and ensure compliance before the new regulations take effect. (Source: CIRS Group)
Sign Up to Receive China Updates Weekly Newsletter for FREE, HERE
Visit HPA-China’s Information Hub, HERE
