In order to help enterprises better understand the filing status of health food (dietary supplements) in China, CIRS Group has gathered statistics on health foods approved in 2024 (as of June 30), and conducted comprehensive analyses from multiple perspectives.
Overview of health food filing status in China
According to the information released by the Special Food Information Query Platform, a total of 2,164 health foods have obtained filing certificates as of June 30, 2024. Among them, 2,143 were domestic health foods and 21 were imported health foods (detailed information on imported health foods is given in Table 1).
Table 1. Filing information on imported health food
| S.N. | Product name | Applicant | Filing number | Country/region |
| 1 | Calcium Citrate | Nutricorp International | 食健备J202400000001 | Canada |
| 2 | Nature’s Way Adult Vita Gummies Calcium+Vitamin D | PHARM-A-CARE LABORATORIES PTY LIMITED | 食健备J202400000002 | Australia |
| 3 | K-Max Calcium Vitamin D and Vitamin K Softgel | Kang Long Group Corporation | 食健备J202400000003 | America |
| 4 | K-Max Vitamin C Tablet Chewable Blueberry flavor | Kang Long Group Corporation | 食健备J202400000004 | America |
| 5 | Vitamin E Softgel | Kang Long Group Corporation | 食健备J202400000005 | America |
| 6 | Calcium Vitamin D Softgel | Kang Long Group Corporation | 食健备J202400000006 | America |
| 7 | Naturies Chewable Vitamin C Tablet | NATURIES HEALTH PRODUCTS LIMITED | 食健备J202400000007 | New Zealand |
| 8 | Naturies Vitamin B Complex Tablet | NATURIES HEALTH PRODUCTS LIMITED | 食健备J202400000008 | New Zealand |
| 9 | Naturies Multi Vitamin&Mineral Tablet (Pregnant Formula) | NATURIES HEALTH PRODUCTS LIMITED | 食健备J202400000009 | New Zealand |
| 10 | Naturies Calcium&Vitamin D Tablet | NATURIES HEALTH PRODUCTS LIMITED | 食健备J202400000010 | New Zealand |
| 11 | Naturies Iron&Folic Acid Tablet | NATURIES HEALTH PRODUCTS LIMITED | 食健备J202400000011 | New Zealand |
| 12 | Naturies Multi Vitamin&Mineral Tablet | NATURIES HEALTH PRODUCTS LIMITED | 食健备J202400000012 | New Zealand |
| 13 | Calcium Plus | Nutra Seasons, LLC | 食健备J202400000013 | America |
| 14 | Zinc Extra | FANCL Corporation | 食健备J202400000014 | Japan |
| 15 | Multiple Vitamin&Mineral Extra W30 | FANCL Corporation | 食健备J202400000015 | Japan |
| 16 | Vitamin B complex Extra | FANCL Corporation | 食健备J202400000016 | Japan |
| 17 | WOTAXEN Vitamin D3 | L’Orientation Biotechnique Canada Inc. | 食健备J202400000017 | Canada |
| 18 | Spray for life®Super B-Complex | NANOSYNERGY WORLDWIDE, INC. | 食健备J202400000018 | America |
| 19 | COLOMBIN TANGERINE VITAMIN C | COLOMBIN Confectionery Co., Ltd. | 食健备J202400000019 | Korea |
| 20 | COLOMBIN CHERRY VITAMIN C | COLOMBIN Confectionery Co., Ltd. | 食健备J202400000020 | Korea |
| 21 | COLOMBIN BLUEBERRY VITAMIN C | COLOMBIN Confectionery Co., Ltd. | 食健备J202400000021 | Korea |
For domestic health foods that have been filed, CIRS will conduct a statistical analysis from four perspectives: regions, enterprises, dosage forms, and functional components.
Health food filing status in different regions
The filing status of domestically produced health foods varies significantly across different regions (provinces, municipalities, and autonomous regions). Shandong Province lead with 540 health food filing certificates. Anhui and Henan followed, with 426 and 242 certificates respectively, ranking the second and the third.
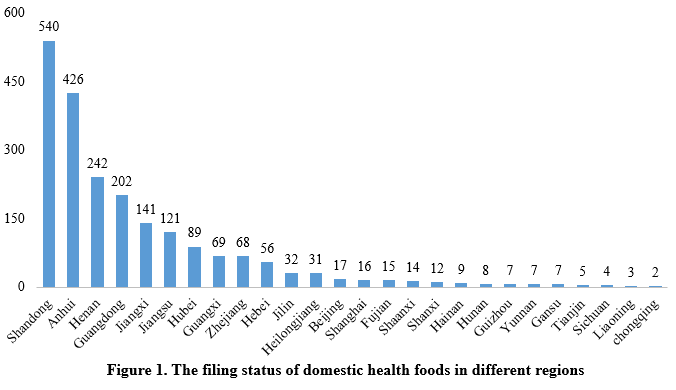
Health food filing status among different enterprises
402 domestic health food enterprises have obtained the filing certificates. Weihai Baihe Biotechnology Co., Ltd. had the largest number of approvals with a total of 207 filed products, followed by Anhui Jinyuan Pharmaceutical Co., Ltd. and Jiangxi Muentang Biotechnology Co., Ltd., with 96 and 57, respectively.

Health food filing status by different dosage forms
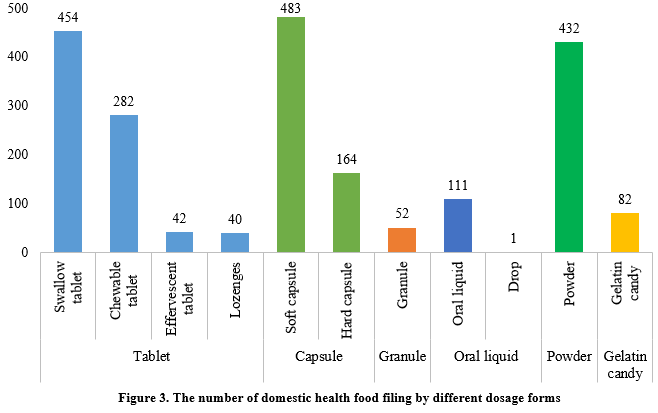
At present, permitted dosage forms for health food filing include tablets, capsules (hard/soft), oral liquids, granules, powders, and gelatin candy (gummies).
In the first half of 2024, the main dosage form for domestic health food filing was tablets, with 818, accounting for 38.2% of the total. Capsule products had a total of 647 filings, covering 483 for soft capsules and 164 for hard capsules. In addition, there were 112 filings for oral liquid (including drops) products and 52 filings for granule products, with 1 drop product among the oral liquid. Powder products and gelatin candy products had 432 and 82 filings, respectively.
Health food filing status by different functional components
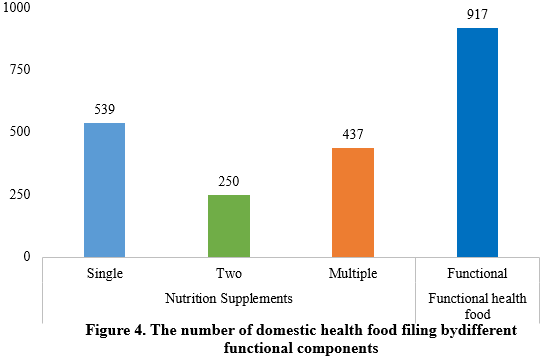
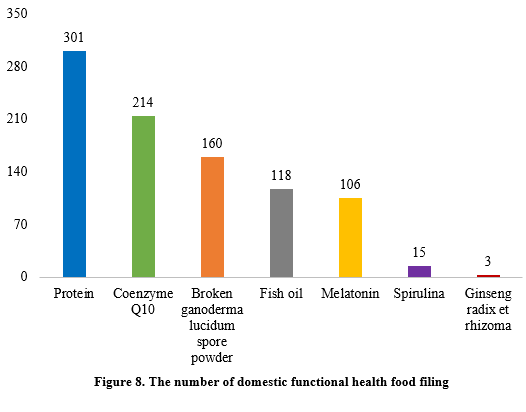
Among the filed domestic health foods in the first half of 2024, there were 1226 nutrition supplements and 917 functional health foods using raw materials such as broken ganoderma lucidum spore powder, accounting for 57.2% and 42.8% of the total, respectively.
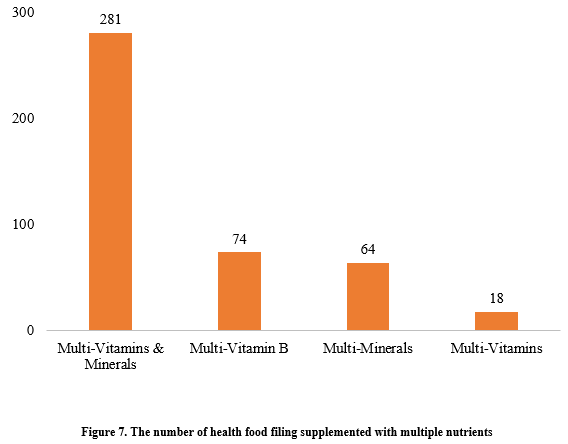
Among the nutrition supplements, products supplementing a single nutrient were greater in quantity, with 539 filings, followed by those supplementing multi-vitamins & minerals (437 products filed) and two nutrients (250 products filed).
Among nutrition supplements, vitamin C supplements, calcium & vitamin D supplements, and multi-vitamins & minerals supplements stood out as the three main product categories, with 201, 107, and 281 filings, respectively. Also gaining prominence were health foods with DHA algal oil as raw material, aimed at supplementing n-3 polyunsaturated fatty acids, ranking third in filings among single-nutrient products.
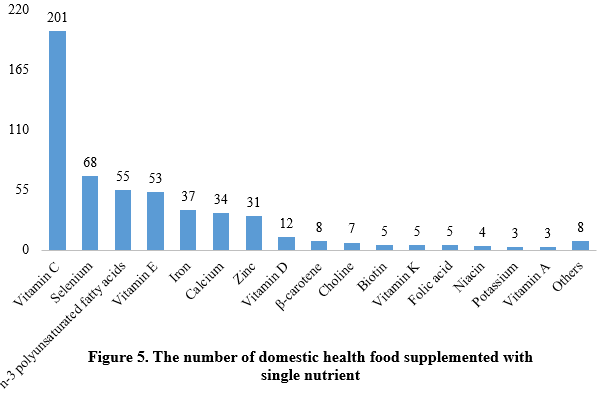
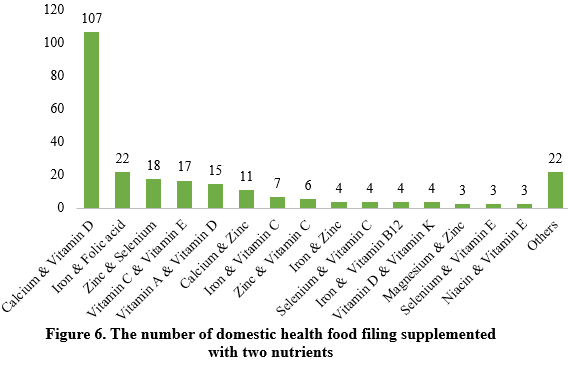
Note: Nutrition supplements with less than three products are categorized as “Others”.
The filing numbers of the functional health food with coenzyme Q10, broken ganoderma lucidum spore powder, fish oil, melatonin, or spirulina as the single raw material were 214, 160, 118, 106, and 15 in the first half of 2024. In addition, the filed products with ginseng radix et rhizome (3 products filed) a single raw material and 301 filed products with protein (soy protein isolate and/or whey protein) as raw materials have been added, of which 20 filed products of protein were combined with vitamins and minerals, with health function being “enhancing immunity”.
CIRS Comments
It is evident from the above analysis that the Chinese health food market has gained new momentum in 2024, with health foods made from DHA algal oil, soy isolate protein, whey protein, and ginseng radix et rhizoma securing filing certificates. At the end of 2023, ginseng radix et rhizome, American ginseng, and Ganoderma lucidum were included in the raw material catalog. On April 30, 2024, the official filing technical requirements were released, and by June 30, health foods made from ginseng radix et rhizoma had obtained registration certificates. It is expected that products made from Ganoderma lucidum or American ginseng will soon emerge in the market. (Source: CIRS Group)
Sign Up to Receive China Updates Weekly Newsletter for FREE, HERE
Visit HPA-China’s Information Hub, HERE
