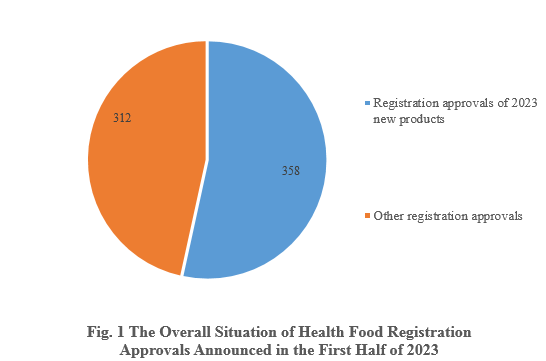
According to the information released by the Center for Food Evaluation, State Administration for Market Regulation and the Special Food Information Query Platform, in the first half of 2023, State Administration for Market Regulation issued a total of 670 health food (dietary supplement) registration approvals, 358 of which are new health food products.
CIRS has summarized these 358 new products, and analyzed them from the following points:
1. Overall Situation
Among the 670 registration approvals, 358 are new products, accounting for 53.43% of the total. Among the other 312 registration approvals, 17 of them are transfer of technology registration, and the rest are possibly renewal of registration and change of registration.
2. The Number of Health Food Registration Approvals in Each Month
As shown in Fig.2, in March, May and June, there are 147, 101 and 74 new products registration approvals released respectively. And there is no new product approvals in the remaining months.
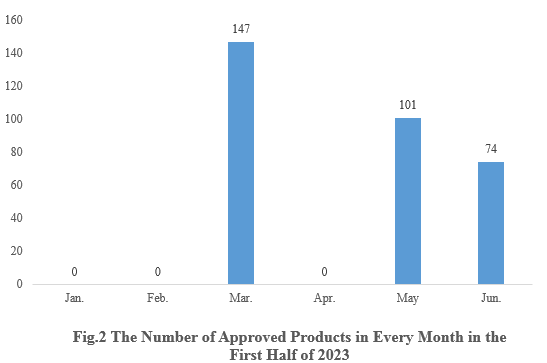
Note 1: There is a lag in the release of approval information, thus 36 products are not able to get the specific approval date. These 36 products are not included in the statistics.
3. The Number of Approved Products in Different Regions
The 358 approved new products are from 28 provinces (municipalities and/or autonomous regions). Beijing, Guangdong and Zhejiang occupy the top three spots with the number of 76, 43 and 31 respectively, accounting for 21.23%, 12.01% and 8.66% of the total new products.
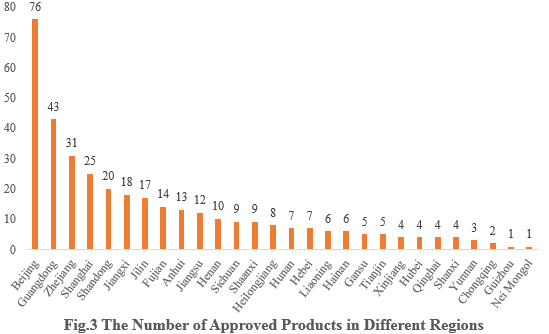
Note 2: If more than one enterprise that are from different provinces have applied for the registrations, all of them are included in the statistics.
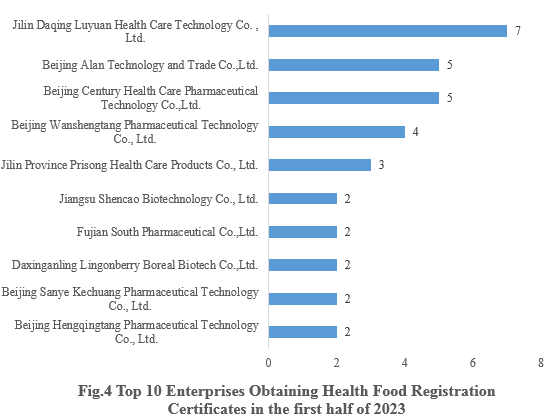
4. Enterprises Obtaining Health Food Registration Certificates
Jilin Daqing Luyuan Health Care Technology Co., Ltd., who obtained 7 health food registration certificates, ranked first in the first half of 2023. The top ten enterprises obtaining new registration certificates in the first half of 2023 are shown in the Fig.4.
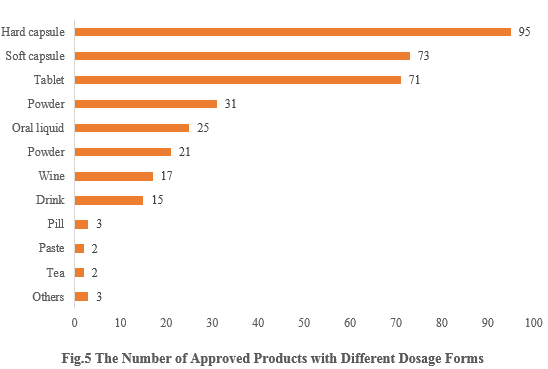
5. The Number of Approved Products with Different Dosage Forms
The dosage forms of approved new products include capsule, tablet, powder, oral liquid, etc. Among them, capsule products received the largest number of approvals, with the number of 168 (including 95 for hard capsule and 73 for soft capsule), accounting for 46.39%. The number of tablet products is 71, accounting for 19.83%.
6. The Number of Approved Products with Different Health Functions
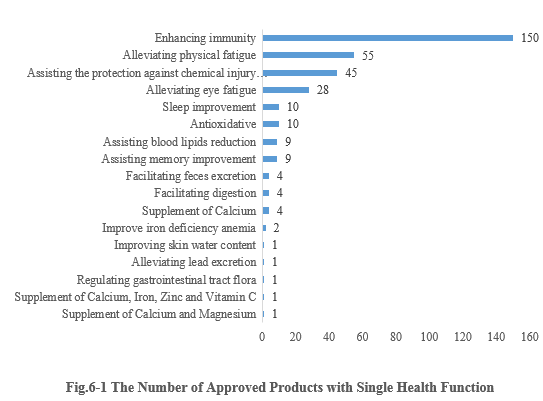
6.1 Single health function
As shown in Fig.6-1, enhancing immunity is the function with the largest number of approvals. The number of new products with the health function of enhancing immunity is 150, accounting for 41.90% of the total new products. Followed by 55 approved products with the health function of alleviating physical fatigue. It’s worth noticing that six nutrient supplement products obtained registration approvals in the first half of this year.
6.2 Two health functions
Except for those new products with single health function, there are also 20 products approved with two health functions (shown in Fig.6-2). It can be seen that most enterprises prefer to choose the function combination of “Enhancing immunity + X” for their products (“X” means other health functions besides enhancing immune). And 9 products with the function combination of “Enhancing immunity & Alleviating physical fatigue” got registration certificates in the first half of 2023.
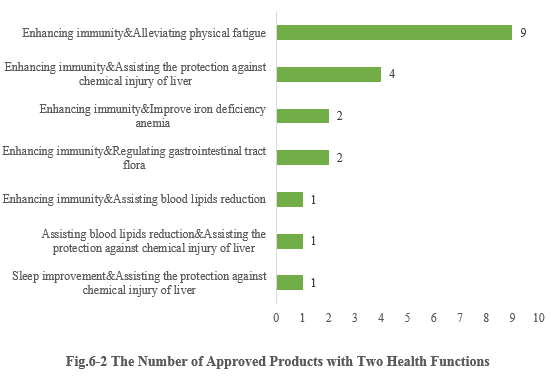
Note 3: Due to the lack of information in the Special Food Information Query Platform, the specific health function of 3 products cannot be found, thus these 3 products are not included in the above statistics.
7. CIRS Comments
Compared with the same period last year, the number of health food new products approved in the half of 2023 increased by 167%, which indicates the review of registered health food products is accelerating, and the health food registration is gradually warming up. From the perspective of the enterprises, CIRS suggests that enterprises focus on the 9 currently available health function evaluation methods before the official release of the new evaluation methods, and make product applications according to consumers’ needs. CIRS has launched a one-stop platform for food ingredients and regulation data -ChinaFoodDB, which aims to provide pre-compliance services for users, including food ingredients inquiry, formula compliance self-check, regulations and announcements acquisition, etc. It is believed to be a powerful tool for enterprises in the process of formula R&D and product innovation. (Source: CIRS Group)
Sign Up to Receive China Updates Weekly Newsletter for FREE, CLICK HERE
Visit HPA-China’s Information Hub, CLICK HERE
