In order to help enterprises better understand the filing status of health food (dietary supplement) in China, CIRS gathered statistics on the health food approved in 2023 (as of June 30) and made an analysis from multiple perspectives.
Filing Status of Health Food in China
According to the information released by the Special Food Information Query Platform, in 2023 (as of June 30), a total of 1969 health food obtained the filing certificates, of which 1953 are domestic health foods and 16 are imported health foods (detailed information is shown in Table 1).
Table 1. Filing Information of Imported Health Food
| S.N. | Product Name | Applicant | Filing Number | Country |
| 1 | 澳琳达牌叶酸胶囊 | TAS MANIA PTY LTD | 食健备J202300000001 | Australia |
| 2 | 澳琳达牌B族维生素片 | TAS MANIA PTY LTD | 食健备J202300000002 | Australia |
| 3 | NZN®多种维生素片 | ALPHA LABORATORIES (NZ) LIMITED | 食健备J202300000003 | New Zealand |
| 4 | 古德海兹牌铁叶酸咀嚼片 | Good Health Products Limited | 食健备J202300000004 | New Zealand |
| 5 | 古德海兹牌钙维生素D软胶囊 | Good Health Products Limited | 食健备J202300000005 | New Zealand |
| 6 | 爱司盟牌叶酸片 | ESMOND NATURAL, INC. | 食健备J202300000006 | America |
| 7 | 爱司盟牌多种矿物质片 | ESMOND NATURAL, INC. | 食健备J202300000007 | America |
| 8 | 康麦斯®维生素C咀嚼片(甜橙味) | Kang Long Group Corporation | 食健备J202300000009 | America |
| 9 | 康麦斯®钙咀嚼片(柠檬味) | Kang Long Group Corporation | 食健备J202300000010 | America |
| 10 | 康麦斯®钙维生素D咀嚼片 | Kang Long Group Corporation | 食健备J202300000011 | America |
| 11 | 澳健莱®锌维生素C片 | AUSNATURES PHARMACEUTICALS PTY LTD | 食健备J202300000012 | Australia |
| 12 | 澳健莱®钙维生素D维生素K片 | AUSNATURES PHARMACEUTICALS PTY LTD | 食健备J202300000013 | Australia |
| 13 | 澳健莱®镁片 | AUSNATURES PHARMACEUTICALS PTY LTD | 食健备J202300000014 | Australia |
| 14 | 安菲斯尔牌钙镁维生素D胶囊 | APEXEL CO., LTD | 食健备J202300000015 | Korea |
| 15 | 澳世康牌B族维生素片 | Nature’s Care Manufacture Pty Limited | 食健备J202300000016 | Australia |
| 16 | 澳世康牌镁锰多种维生素胶囊 | Nature’s Care Manufacture Pty Limited | 食健备J202300000017 | Australia |
Filing Status of Health Food in Different Regions
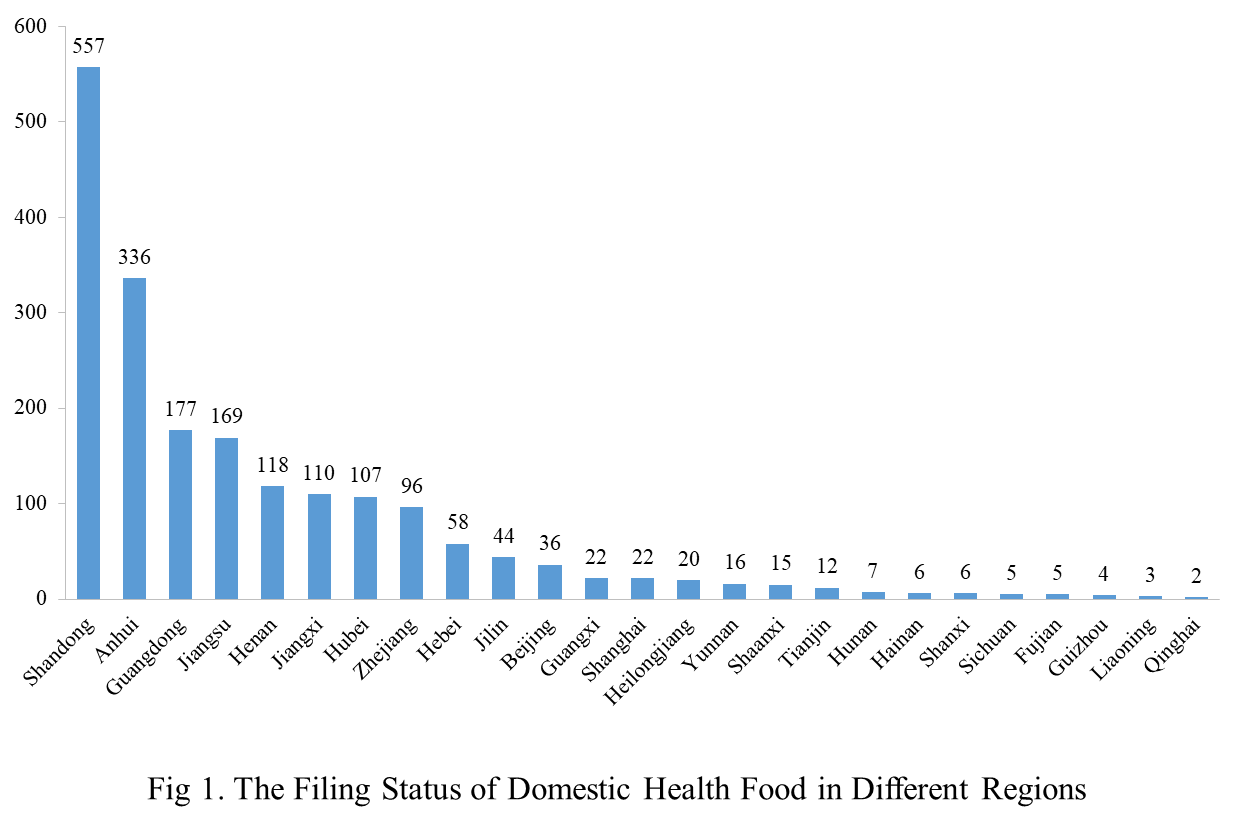
The number of approved domestic filing health food is varied in different regions in China. Shandong has the largest number of filings, and a total of 557 products obtained the filing certificates. Anhui and Guangdong rank the second and third place respectively with the number of 336 and 177.
Filing Status of Health Food in Different Enterprises
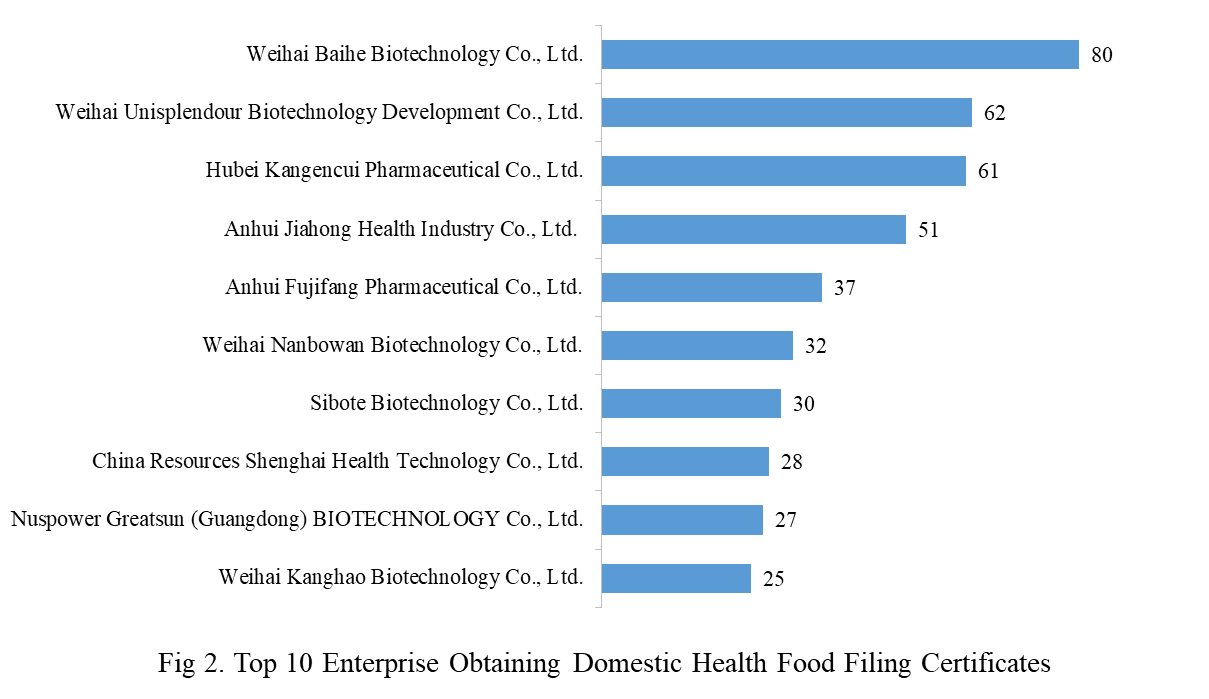
A number of 403 domestic health food manufacturers have obtained health food filing certificates. Weihai Baihe Biotechnology Co., Ltd. has the largest number of approvals with a total of 80 filed products, followed by Weihai Unisplendour Biotechnology Development Co., Ltd. and Hubei Kangencui Pharmaceutical Co., Ltd., with the number of 62 and 61, respectively.
Filing Status of Health Food in Different Dosage Forms
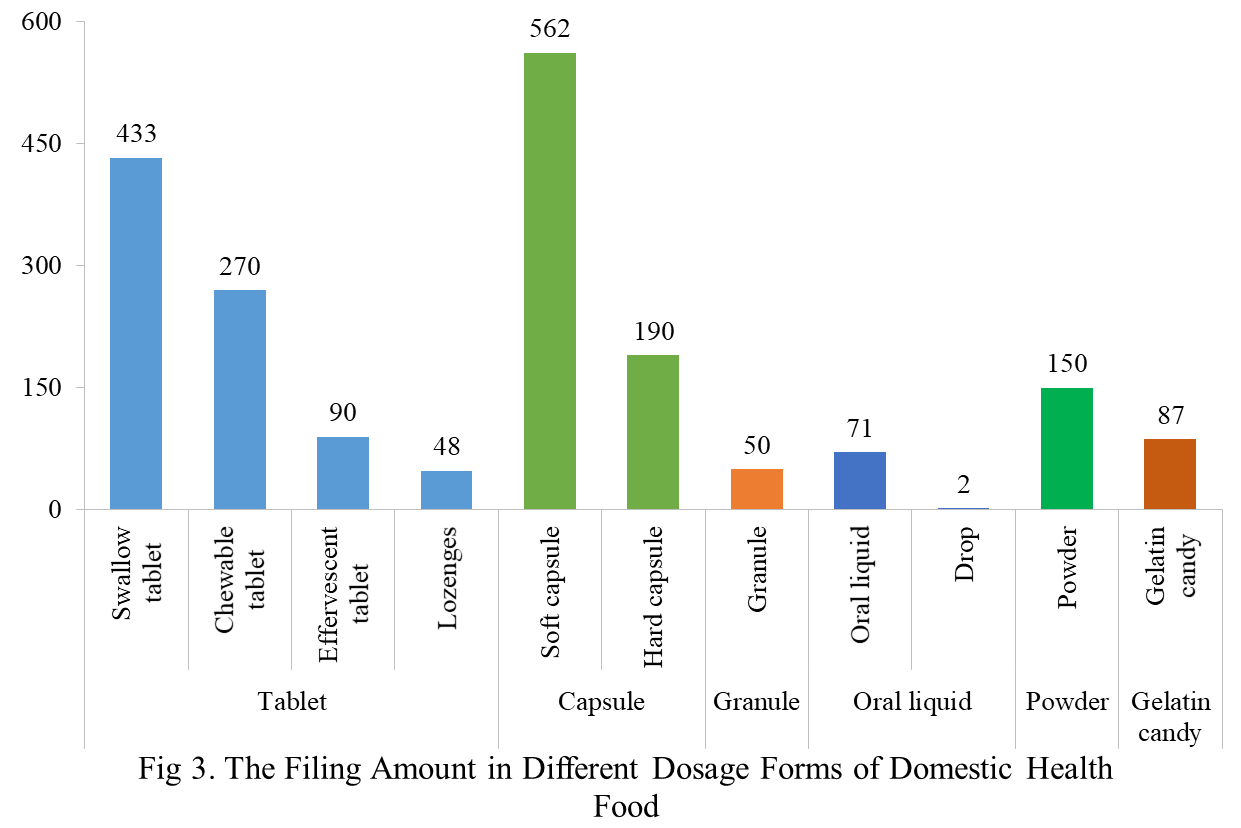
At present, the permitted dosage forms for filing include tablets, capsules (hard/soft), oral liquids, granules, powders and gelatin candy (gummies).
The main dosage form for domestic health food filing is tablets with the number of 841, accounting for 43.1% of the total. The capsules include soft capsules and hard capsules, and the number of filings is 752. The quantity of soft capsule products is far higher than that of hard capsule products, which are 562 and 190 respectively. In addition, the quantity of oral liquids (including drops) and granule products are 73 and 50 respectively. Among oral liquid products, there are only 2 drop products. The quantity of powder and gelatin candy (gummies) products are 150 and 87 respectively.
Filing Status of Health Food in Different Functional Ingredients
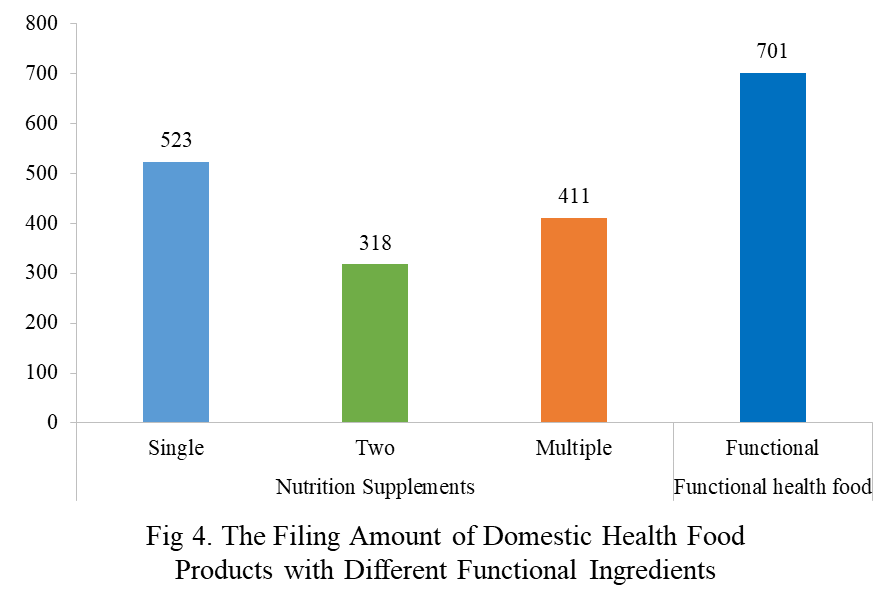
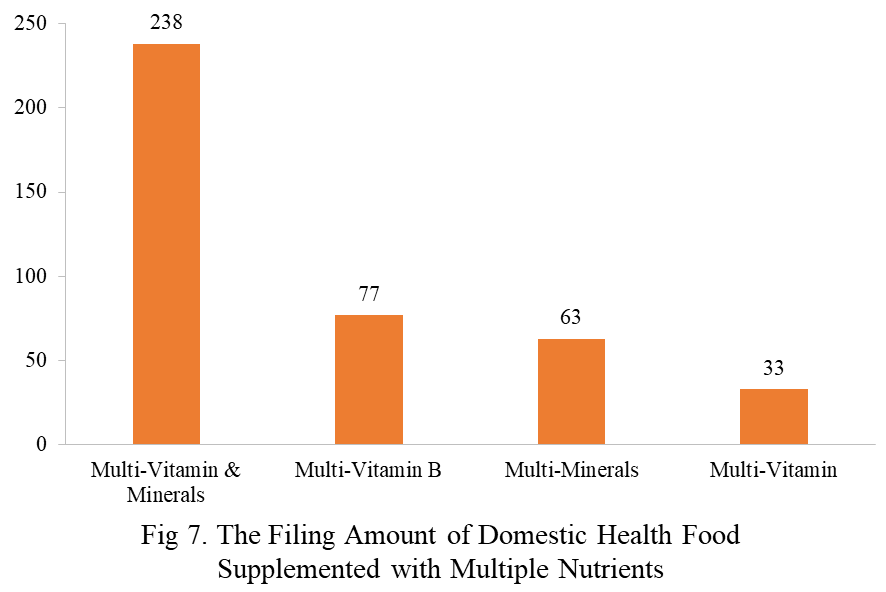
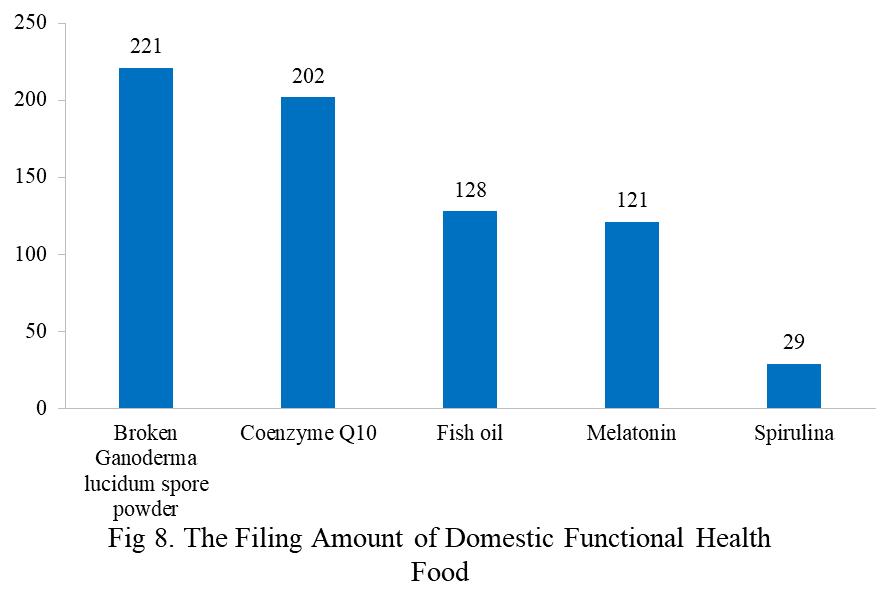
Among the filed domestic health foods in 2023, there are 1252 nutrition supplements, accounting for 64.1% of the total. The rests are 701 functional health food with broken ganoderma lucidum spore powder, coenzyme Q10, melatonin, fish oil or spirulina as single raw material. Among the nutrition supplements, products with the function of supplementing a single nutrient are greater in quantity, whose number is 523, followed by products supplementing multi-vitamins & minerals (411 products are filed) and two nutrients (318 products are filed).
Among the domestic filed products, the most popular nutrition supplements are Vitamin C supplements, Calcium & Vitamin D supplements and Multi-vitamins & minerals supplements, the number of which are 221, 132 and 238 respectively.
The functional health food with broken ganoderma lucidum spore powder, coenzyme Q10, fish oil, melatonin or spirulina as the single raw material has been transferred to filing supervision on June 1, 2021. Their filing numbers are 221, 202, 128, 121 and 29 in 2023 (as of June 30).
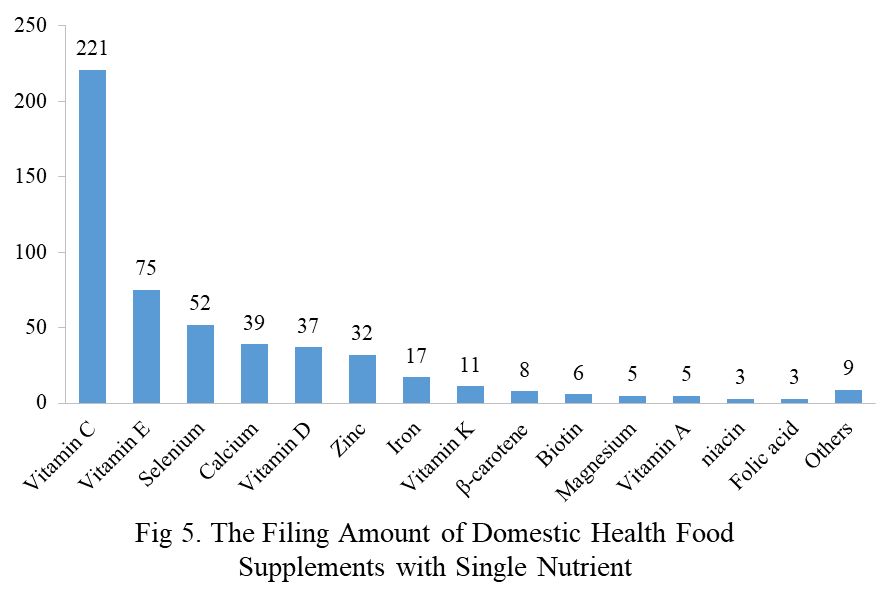
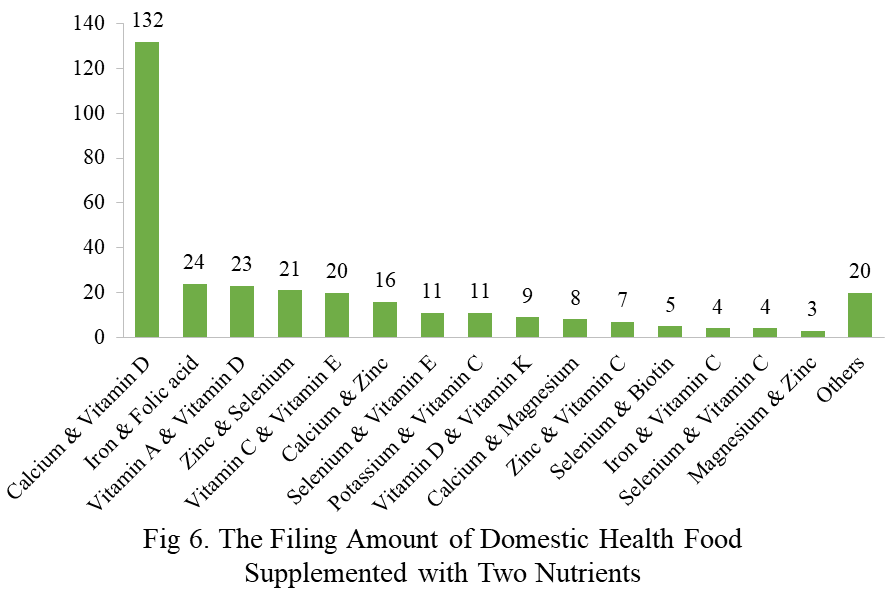
Note: Nutrition supplements with less than 3 products are classified as “Others”.
CIRS Comments
On 14 June 2023, State Administration for Market Regulation (SAMR) issued four new regulations, including,
- Health Food Raw Materials Directory of Nutrition Supplements (2023 Version),
- Health Function Directory of Allowing Nutrition Supplement Claims (2023 Version),
- Health Food Raw Materials Directory – Soy Protein Isolate, and
- Health Food Raw Materials Directory – Whey Protein
These four regulations will take effect from October 1, 2023. Compared with the current Raw Materials Directory, “DHA” is added to the Raw Materials Directory (2023 Version). As the function of this nutrient (“supplying n-3 polyunsaturated fatty acids”) is within the scope of “supply nutrients”, CIRS believes that overseas enterprises can also apply for the filing of DHA products. In addition, soy protein isolate and whey protein have become newly available raw materials for health food filing, whose filing scope of health food made of the two raw materials is expected to be applicable for domestic products only.
We believe that in the future, with the continuous expansion of the directory of health food raw materials and improvement of the dual-track system of health food, there will be more health food categories into the public view, injecting vitality into the Chinese market. (Source: cirs-group)
Sign Up to Receive China Updates Weekly Newsletter for FREE, CLICK HERE
Visit HPA-China’s Information Hub, CLICK HERE
